Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
#1 Dark Discussions at Cafe Infinity » Children Quotes - XIX » Yesterday 00:03:25
- Jai Ganesh
- Replies: 0
Children Quotes - XIX
1. A revolution can be neither made nor stopped. The only thing that can be done is for one of several of its children to give it a direction by dint of victories. - Napoleon Bonaparte
2. The best way to give advice to your children is to find out what they want and then advise them to do it. - Harry S Truman
3. The thing I want more than anything else? I want to have children. I used to feel for every child I had, I would adopt another. - Marilyn Monroe
4. It is good to realize that if love and peace can prevail on earth, and if we can teach our children to honor nature's gifts, the joys and beauties of the outdoors will be here forever. - Jimmy Carter
5. It was always going to be difficult, no matter what I'd set up, no matter how many children I have got to take my mind off things. There was always going to be a moment when I finished playing, that I was going to find tough. - David Beckham
6. It is good to realize that if love and peace can prevail on earth, and if we can teach our children to honor nature's gifts, the joys and beauties of the outdoors will be here forever. - Jimmy Carter
7. I have very good executives and great children. They're very good. - Donald Trump
8. When I see my children, and when I see the people who value me, I know how lucky I am. - Kevin Costner
9. I never thought I'd have children; I never thought I'd be in love, I never thought I'd meet the right person. Having come from a broken home - you kind of accept that certain things feel like a fairy tale, and you just don't look for them. - Angelina Jolie
10. If children grew up according to early indications, we should have nothing but geniuses. - Johann Wolfgang von Goethe
11. Parentage is a very important profession, but no test of fitness for it is ever imposed in the interest of the children. - George Bernard Shaw
12. I can tell you in all honesty that I am highly connected to my family, my wife, and my three children, though I don't get to spend dollops of hours with them. - Ratan Tata
13. If the whole of mankind is to be united into one brotherhood, all obstacles must be removed so that men, all over the surface of the globe, should be as children playing in a garden. - Maria Montessori
14. Unlike grown ups, children have little need to deceive themselves. - Johann Wolfgang von Goethe.
#2 Jokes » Arithmetic Jokes - II » 2024-11-25 23:26:01
- Jai Ganesh
- Replies: 0
Q: What do you call a fish that knows addition?
A: An Octoplus.
* * *
Q: Why is an math book always unhappy?
A: Because it always has lots of problems.
* * *
Q: What do you get if you add two apples and three apples?
A: A grade school math problem!
* * *
Q: Why did the two 4's skip lunch?
A: They already 8 (ate)!
* * *
Q: Why can't you put 8 and 2 next to each other in math class?
A: Because they don't pay at10ention.
* * *
#3 Re: Jai Ganesh's Puzzles » 10 second questions » 2024-11-25 15:36:36
Hi,
#9393.
#4 Re: Jai Ganesh's Puzzles » Oral puzzles » 2024-11-25 15:29:33
Hi,
#5909.
#5 Re: Exercises » Compute the solution: » 2024-11-25 14:55:42
Hi,
2240.
#6 Re: This is Cool » Attention Deficit Hyperactivity Disorder (ADHD) » 2024-11-25 13:21:14
Hi Coralie L,
Thanks!
#7 Re: This is Cool » Miscellany » 2024-11-24 22:46:37
2270) Pharmacology
Gist
Pharmacology is the science of how drugs act on biological systems and how the body responds to the drug. The study of pharmacology encompasses the sources, chemical properties, biological effects and therapeutic uses of drugs.
Pharmacology is the study of the effects of drugs on living organisms where a drug can be broadly defined as any chemical substance, natural or synthetic, that affects a biological system.
Summary
Pharmacology is the science of drugs and medications, including a substance's origin, composition, pharmacokinetics, pharmacodynamics, therapeutic use, and toxicology. More specifically, it is the study of the interactions that occur between a living organism and chemicals that affect normal or abnormal biochemical function. If substances have medicinal properties, they are considered pharmaceuticals.
The field encompasses drug composition and properties, functions, sources, synthesis and drug design, molecular and cellular mechanisms, organ/systems mechanisms, signal transduction/cellular communication, molecular diagnostics, interactions, chemical biology, therapy, and medical applications and antipathogenic capabilities. The two main areas of pharmacology are pharmacodynamics and pharmacokinetics. Pharmacodynamics studies the effects of a drug on biological systems, and pharmacokinetics studies the effects of biological systems on a drug. In broad terms, pharmacodynamics discusses the chemicals with biological receptors, and pharmacokinetics discusses the absorption, distribution, metabolism, and excretion (ADME) of chemicals from the biological systems.
Pharmacology is not synonymous with pharmacy and the two terms are frequently confused. Pharmacology, a biomedical science, deals with the research, discovery, and characterization of chemicals which show biological effects and the elucidation of cellular and organismal function in relation to these chemicals. In contrast, pharmacy, a health services profession, is concerned with the application of the principles learned from pharmacology in its clinical settings; whether it be in a dispensing or clinical care role. In either field, the primary contrast between the two is their distinctions between direct-patient care, pharmacy practice, and the science-oriented research field, driven by pharmacology.
Details
Pharmacology is the branch of medicine that deals with the interaction of drugs with the systems and processes of living animals, in particular, the mechanisms of drug action as well as the therapeutic and other uses of the drug.
The first Western pharmacological treatise, a listing of herbal plants used in classical medicine, was made in the 1st century ad by the Greek physician Dioscorides. The medical discipline of pharmacology derives from the medieval apothecaries, who both prepared and prescribed drugs. In the early 19th century a split developed between apothecaries who treated patients and those whose interest was primarily in the preparation of medicinal compounds; the latter formed the basis of the developing specialty of pharmacology. A truly scientific pharmacology developed only after advances in chemistry and biology in the late 18th century enabled drugs to be standardized and purified. By the early 19th century, French and German chemists had isolated many active substances—morphine, strychnine, atropine, quinine, and many others—from their crude plant sources. Pharmacology was firmly established in the later 19th century by the German Oswald Schmeiderberg (1838–1921). He defined its purpose, wrote a textbook of pharmacology, helped to found the first pharmacological journal, and, most importantly, headed a school at Strasbourg that became the nucleus from which independent departments of pharmacology were established in universities throughout the world. In the 20th century, and particularly in the years since World War II, pharmacological research has developed a vast array of new drugs, including antibiotics, such as penicillin, and many hormonal drugs, such as insulin and cortisone. Pharmacology is presently involved in the development of more effective versions of these and a vast array of other drugs through chemical synthesis in the laboratory. Pharmacology also seeks more efficient and effective ways of administering drugs through clinical research on large numbers of patients.
During the early 20th century, pharmacologists became aware that a relation exists between the chemical structure of a compound and the effects it produces in the body. Since that time, increasing emphasis has been placed on this aspect of pharmacology, and studies routinely describe the changes in drug action resulting from small changes in the chemical structure of the drug. Because most medical compounds are organic chemicals, pharmacologists who engage in such studies must necessarily have an understanding of organic chemistry.
Important basic pharmacological research is carried out in the research laboratories of pharmaceutical and chemical companies. After 1930 this area of pharmacological research underwent a vast and rapid expansion, particularly in the United States and Europe.
The work of pharmacologists in industry deals also with the exhaustive tests that must be made before promising new drugs can be introduced into medical use. Detailed observations of a drug’s effects on all systems and organs of laboratory animals are necessary before the physician can accurately predict both the effects of the drug on patients and their potential toxicity to humans in general. The pharmacologist does not himself test the effects of drugs in patients; this is done only after exhaustive tests on animals and is usually conducted by physicians to determine the clinical effectiveness of new drugs. Constant testing is also required for the routine control and standardization of drug products and their potency and purity.
Additional Information
Pharmacology is the scientific study of the effects of drugs and chemicals on living organisms where a drug can be broadly defined as any chemical substance, natural or synthetic, which affects a biological system. Pharmacology may involve how organisms handle drugs, identification and validation of new targets for drug action, and the design and development of new drugs to prevent, treat and cure disease.
Pharmacology research is also a critical component in the development of modern 'personalized medicine'.
There are many sub-specialties within the general discipline of pharmacology. Pharmacodynamics is the study of the effects of drugs on biological systems and specifically addresses the chemical properties and physiological and behavioral effects of drugs arising from their interaction with molecular targets such as receptor proteins or enzyme systems. In contrast, pharmacokinetics is the study of what biological systems do to the drug and encompasses investigations of drug absorption, distribution, biotransformation and excretion, essential information for the design of drug treatment schedules in different patient populations and experimental animals, and for the prediction of drug-drug interactions that may enhance or compromise the effectiveness and safety of therapeutic agents.
Pharmacologists require sound basic knowledge of physiology, biochemistry, cell biology and molecular biology upon which to build their specialized knowledge and experimental approaches for the investigation of novel aspects of drug action. Such studies may occur at various levels, including molecular interactions, cellular and subcellular signal transduction processes, tissue and organ regulation, as well as integrated physiological or behavioral responses in intact organisms. The knowledge acquired facilitates the development of new drugs and contributes to rational therapeutics that involves the safe and effective use of drugs for therapeutic benefit. Also, this interdisciplinary knowledge offers pharmacologists a unique perspective on a wide range of biomedical issues and enhances employment opportunities in many areas of scientific investigation.
While pharmacologists are trained as laboratory researchers, pharmacists usually work in a hospital or retail pharmacy and are concerned with the preparation, dispensing, dosage, and the safe and effective use of therapeutic agents.

#8 Dark Discussions at Cafe Infinity » Children Quotes - XVIII » 2024-11-24 22:16:18
- Jai Ganesh
- Replies: 0
Children Quotes - XVIII
1. I don't mind fans coming up in a friendly, respectful way. That's all part of the fun of being a top tennis player. But if people take pictures without permission, particularly if my children are in the shot, I feel uncomfortable. - Roger Federer
2. Those who educate children well are more to be honored than they who produce them; for these only gave them life, those the art of living well. - Aristotle
3. Creativity is the key to success in the future, and primary education is where teachers can bring creativity in children at that level. - A. P. J. Abdul Kalam
4. If help and salvation are to come, they can only come from the children, for the children are the makers of men. - Maria Montessori
5. For in the final analysis, our most basic common link is that we all inhabit this small planet. We all breathe the same air. We all cherish our children's futures. And we are all mortal. - John F. Kennedy
6. Without education, your children can never really meet the challenges they will face. So it's very important to give children education and explain that they should play a role for their country. - Nelson Mandela
7. No man should bring children into the world who is unwilling to persevere to the end in their nature and education. - Plato
8. For unflagging interest and enjoyment, a household of children, if things go reasonably well, certainly all other forms of success and achievement lose their importance by comparison. - Theodore Roosevelt.
#9 Jokes » Arithmetic Jokes - I » 2024-11-24 21:48:24
- Jai Ganesh
- Replies: 0
Q: Why do they never serve beer at a math party?
A: Because you can't drink and derive...
* * *
Q: Why didn't the quarter roll down the hill with the nickel?
A: Because it had more cents.
* * *
Q: What happened to the plant in math class?
A: It grew square roots.
* * *
Q: How do you make seven an even number?
A: Take the s out!
* * *
Q: Why wasn't the geometry teacher at school?
A: Because she sprained her angle!!
* * *
#10 Re: Jai Ganesh's Puzzles » 10 second questions » 2024-11-24 15:03:49
Hi,
#9392.
#11 Re: Jai Ganesh's Puzzles » Oral puzzles » 2024-11-24 14:32:12
Hi,
#5908.
#12 Re: Exercises » Compute the solution: » 2024-11-24 14:10:19
Hi,
2239.
#13 Science HQ » pH Meter » 2024-11-23 22:46:31
- Jai Ganesh
- Replies: 0
pH Meter
Gist
What does a pH meter measure? An electronic pH meter is used to obtain more accurate pH measurements. A pH meter is an instrument used to measure hydrogen ion activity in solutions - in other words, this instrument measures acidity/alkalinity of a solution.
Summary
pH meter is an electric device used to measure hydrogen-ion activity (acidity or alkalinity) in solution. Fundamentally, a pH meter consists of a voltmeter attached to a pH-responsive electrode and a reference (unvarying) electrode. The pH-responsive electrode is usually glass, and the reference is usually a silver–silver chloride electrode, although a mercury–mercurous chloride (calomel) electrode is sometimes used. When the two electrodes are immersed in a solution, they act as a battery. The glass electrode develops an electric potential (charge) that is directly related to the hydrogen-ion activity in the solution (59.2 millivolts per pH unit at 25 °C [77 °F]), and the voltmeter measures the potential difference between the glass and reference electrodes.
Details
A pH meter is a scientific instrument that measures the hydrogen-ion activity in water-based solutions, indicating its acidity or alkalinity expressed as pH. The pH meter measures the difference in electrical potential between a pH electrode and a reference electrode, and so the pH meter is sometimes referred to as a "potentiometric pH meter". The difference in electrical potential relates to the acidity or pH of the solution. Testing of pH via pH meters (pH-metry) is used in many applications ranging from laboratory experimentation to quality control.
Applications:
The rate and outcome of chemical reactions taking place in water often depends on the acidity of the water, and it is therefore useful to know the acidity of the water, typically measured by means of a pH meter. Knowledge of pH is useful or critical in many situations, including chemical laboratory analyses. pH meters are used for soil measurements in agriculture, water quality for municipal water supplies, swimming pools, environmental remediation; brewing of wine or beer; manufacturing, healthcare and clinical applications such as blood chemistry; and many other applications.
Advances in the instrumentation and in detection have expanded the number of applications in which pH measurements can be conducted. The devices have been miniaturized, enabling direct measurement of pH inside of living cells. In addition to measuring the pH of liquids, specially designed electrodes are available to measure the pH of semi-solid substances, such as foods. These have tips suitable for piercing semi-solids, have electrode materials compatible with ingredients in food, and are resistant to clogging.
Design and use:
Principle of operation
Potentiometric pH meters measure the voltage between two electrodes and display the result converted into the corresponding pH value. They comprise a simple electronic amplifier and a pair of electrodes, or alternatively a combination electrode, and some form of display calibrated in pH units. It usually has a glass electrode and a reference electrode, or a combination electrode. The electrodes, or probes, are inserted into the solution to be tested. pH meters may also be based on the antimony electrode (typically used for rough conditions) or the quinhydrone electrode.
In order to accurately measure the potential difference between the two sides of the glass membrane reference electrode, typically a silver chloride electrode or calomel electrode are required on each side of the membrane. Their purpose is to measure changes in the potential on their respective side. One is built into the glass electrode. The other, which makes contact with the test solution through a porous plug, may be a separate reference electrode or may be built into a combination electrode. The resulting voltage will be the potential difference between the two sides of the glass membrane possibly offset by some difference between the two reference electrodes, that can be compensated for.
The design of the electrodes is the key part: These are rod-like structures usually made of glass, with a bulb containing the sensor at the bottom. The glass electrode for measuring the pH has a glass bulb specifically designed to be selective to hydrogen-ion concentration. On immersion in the solution to be tested, hydrogen ions in the test solution exchange for other positively charged ions on the glass bulb, creating an electrochemical potential across the bulb. The electronic amplifier detects the difference in electrical potential between the two electrodes generated in the measurement and converts the potential difference to pH units. The magnitude of the electrochemical potential across the glass bulb is linearly related to the pH according to the Nernst equation.
The reference electrode is insensitive to the pH of the solution, being composed of a metallic conductor, which connects to the display. This conductor is immersed in an electrolyte solution, typically potassium chloride, which comes into contact with the test solution through a porous ceramic membrane. The display consists of a voltmeter, which displays voltage in units of pH.
On immersion of the glass electrode and the reference electrode in the test solution, an electrical circuit is completed, in which there is a potential difference created and detected by the voltmeter. The circuit can be thought of as going from the conductive element of the reference electrode to the surrounding potassium-chloride solution, through the ceramic membrane to the test solution, the hydrogen-ion-selective glass of the glass electrode, to the solution inside the glass electrode, to the silver of the glass electrode, and finally the voltmeter of the display device. The voltage varies from test solution to test solution depending on the potential difference created by the difference in hydrogen-ion concentrations on each side of the glass membrane between the test solution and the solution inside the glass electrode. All other potential differences in the circuit do not vary with pH and are corrected for by means of the calibration.
For simplicity, many pH meters use a combination probe, constructed with the glass electrode and the reference electrode contained within a single probe.
The pH meter is calibrated with solutions of known pH, typically before each use, to ensure accuracy of measurement. To measure the pH of a solution, the electrodes are used as probes, which are dipped into the test solutions and held there sufficiently long for the hydrogen ions in the test solution to equilibrate with the ions on the surface of the bulb on the glass electrode. This equilibration provides a stable pH measurement.

#14 Re: Dark Discussions at Cafe Infinity » crème de la crème » 2024-11-23 21:43:56
2100) Peter Grünberg
Gist:
Work
When materials are reduced to just a few atomic layers—a few nanometers in thickness—their properties change. Independently of one another, Peter Grünberg and Albert Fert discovered the phenomenon Giant Magneto Resistance (GMR) in 1988. GMR involves small changes in magnetic fields creating major differences in electrical resistance. It has also had an impact on electronics, especially read heads, where information stored magnetically is converted to electric current. Thanks to GMR, hard drives have become much smaller.
Summary
Peter Grünberg (born May 18, 1939, Plzeň, Czechoslovakia [now in the Czech Republic]—died April 2018, Jülich, Germany) was a Czech-born German scientist who, with Albert Fert, received the 2007 Nobel Prize for Physics for his independent codiscovery of giant magnetoresistance.
Grünberg completed his undergraduate studies in physics in 1962 at Johann Wolfgang Goethe University in Frankfurt am Main, Ger. He was awarded a master’s degree (1966) and doctorate (1969) by the Darmstadt University of Technology. From 1972 until his retirement in 2004, he was a research scientist at the Institute of Solid State Research at the Helmholtz Research Centre in Jülich, Ger.
In 1857 Lord Kelvin was the first to observe that it was possible to alter the electric resistance of a conducting material by placing it in an external magnetic field. Grünberg expanded on this principle by placing a layer of nonmagnetic metal between layers of magnetized metal to create an effect that Fert termed giant magnetoresistance. By changing the direction of magnetization within the system, the resistance could be greatly increased or reduced. The practical application of this phenomenon was an exponential expansion in the capacity of magnetic storage devices such as computer hard drives.
Details
Peter Andreas Grünberg (18 May 1939 – 7 April 2018) was a German physicist, and Nobel Prize in Physics laureate for his discovery with Albert Fert of giant magnetoresistance which brought about a breakthrough in gigabyte hard disk drives.
Life and career
Grünberg was born in Plzeň, Czechoslovakia, which at the time was known as Pilsen in the German-occupied Protectorate of Bohemia and Moravia (now the Czech Republic) to the Sudeten German family of Anna and Feodor A. Grünberg. They first lived in Dýšina to the east of Plzeň. Grünberg was a Catholic.
After the war, the family was interned; the parents were brought to a camp. His father, a Russia-born engineer who since 1928 had worked for Škoda, died on 27 November 1945 in Czech imprisonment and is buried in a mass grave in Plzeň which is also inscribed with Grünberg Theodor † 27. November 1945. His mother Anna (who died in 2002 aged 100) had to work in agriculture and stayed with her parents in the Petermann house in Untersekerschan (Dolní Sekyřany), where her children (Peter's sister was born in 1937) were brought later. The remaining Grünberg family, like almost all Germans, was expelled from Czechoslovakia in 1946. Seven-year-old Peter came to Lauterbach, Hesse where he attended gymnasium.
Grünberg received his intermediate diploma in 1962 from the Johann Wolfgang Goethe University in Frankfurt. He then attended the Technische Universität Darmstadt, where he received his BSc diploma in physics in 1966 and his Ph.D. in 1969. While there, he met and married his wife, Helma Prauser, who became a schoolteacher. From 1969 to 1972, he did postdoctoral work at Carleton University in Ottawa, Canada. He later joined the Institute for Solid State Physics at Forschungszentrum Jülich, Jülich, Germany, where he became a leading researcher in the field of thin film and multilayer magnetism until his retirement in 2004.
In 1984–1985 he served as visiting scientist at Argonne National Laboratories, Lemont, Illinois, USA. From 1984 to 1992 he had Habilitation process and was a lecturer (Junior Professor), and since 1992 till 2004 a Tenured Professor (ausserplanmässiger Professor) at the University of Cologne, Germany. He was also a visiting professor at the Tohoku University at Sendai-shi, Miyagi-ken, Japan from 1998 till 2004.
In 2007, Grünberg was awarded Honorary Doctorate from the RWTH Aachen University, Aachen, Germany, in 2008 Honorary Doctorate from the Saarland University, and from Gebze Institute of Technology, and in 2009 from the University of Athens.
Important work
In 1986 he discovered the antiparallel exchange coupling between ferromagnetic layers separated by a thin non-ferromagnetic layer, and in 1988 he discovered the giant magnetoresistive effect (GMR). GMR was simultaneously and independently discovered by Albert Fert from the Université de Paris Sud. It has been used extensively in read heads of modern hard drives. Another application of the GMR effect is non-volatile, magnetic random access memory.
Apart from the Nobel Prize, work also has been rewarded with shared prizes in the APS International Prize for New Materials, the International Union of Pure and Applied Physics Magnetism Award, the Hewlett-Packard Europhysics Prize, the Wolf Prize in Physics and the 2007 Japan Prize. He won the German Future Prize for Technology and Innovation in 1998 and was named European Inventor of the Year in the category "Universities and research institutions" by the European Patent Office and European Commission in 2006.

#15 Re: This is Cool » Miscellany » 2024-11-23 20:14:39
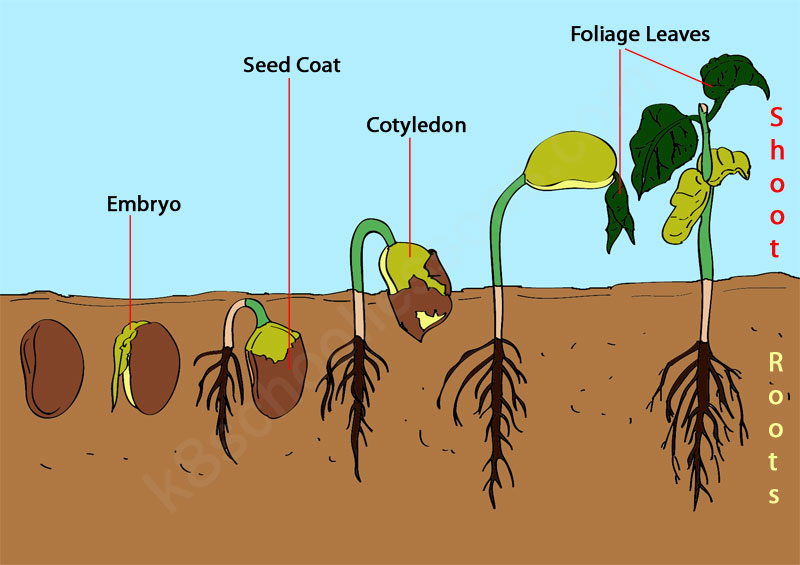
2269) Germination
Gist
Germination is the process by which a plant grows from a seed into a seedling. Seeds remain dormant until conditions are favorable for germination. All seeds need water, oxygen and optimal temperature to germinate.
Summary
Germination is the process by which an organism grows from a seed or spore. The term is applied to the sprouting of a seedling from a seed of an angiosperm or gymnosperm, the growth of a sporeling from a spore, such as the spores of fungi, ferns, bacteria, and the growth of the pollen tube from the pollen grain of a seed plant.
Seed plants
Germination is usually the growth of a plant contained within a seed resulting in the formation of the seedling. It is also the process of reactivation of metabolic machinery of the seed resulting in the emergence of radicle and plumule. The seed of a vascular plant is a small package produced in a fruit or cone after the union of male and female reproductive cells. All fully developed seeds contain an embryo and, in most plant species some store of food reserves, wrapped in a seed coat. Dormant seeds are viable seeds that do not germinate because they require specific internal or environmental stimuli to resume growth. Under proper conditions, the seed begins to germinate and the embryo resumes growth, developing into a seedling.
Step 1: Water imbibition, the uptake of water, results in rupture of seed coat.
Step 2: The imbibition of the seed coat results in emergence of the radicle (1) and the plumule (2); the cotyledons are unfolded (3).
Step 3: This marks the final step in the germination of the seed, where the cotyledons are expanded, which are the true leaves. Note: Temperature must be kept at an optimum level.
Disturbance of soil can result in vigorous plant growth by exposing seeds already in the soil to changes in environmental factors where germination may have previously been inhibited by depth of the seeds or soil that was too compact. This is often observed at gravesites after a burial.
Seed germination depends on both internal and external conditions. The most important external factors include right temperature, water, oxygen or air and sometimes light or darkness. Various plants require different variables for successful seed germination. Often this depends on the individual seed variety and is closely linked to the ecological conditions of a plant's natural habitat. For some seeds, their future germination response is affected by environmental conditions during seed formation; most often these responses are types of seed dormancy.
* Water is required for germination. Mature seeds are often extremely dry and need to take in significant amounts of water, relative to the dry weight of the seed, before cellular metabolism and growth can resume. Most seeds need enough water to moisten the seeds but not enough to soak them. The uptake of water by seeds is called imbibition, which leads to the swelling and the breaking of the seed coat. When seeds are formed, most plants store a food reserve with the seed, such as starch, proteins, or oils. This food reserve provides nourishment to the growing embryo. When the seed imbibes water, hydrolytic enzymes are activated which break down these stored food resources into metabolically useful chemicals. After the seedling emerges from the seed coat and starts growing roots and leaves, the seedling's food reserves are typically exhausted; at this point photosynthesis provides the energy needed for continued growth and the seedling now requires a continuous supply of water, nutrients, and light.
* Oxygen is required by the germinating seed for metabolism. Oxygen is used in aerobic respiration, the main source of the seedling's energy until it grows leaves. Oxygen is an atmospheric gas that is found in soil pore spaces; if a seed is buried too deeply within the soil or the soil is waterlogged, the seed can be oxygen starved. Some seeds have impermeable seed coats that prevent oxygen from entering the seed, causing a type of physical dormancy which is broken when the seed coat is worn away enough to allow gas exchange and water uptake from the environment.
In a small number of plants, such as rice, anaerobic germination can occur in waterlogged conditions. The seed produces a hollow coleoptile that acts like a 'snorkel', providing the seed with access to oxygen.
* Temperature affects cellular metabolic and growth rates. Seeds from different species and even seeds from the same plant germinate over a wide range of temperatures. Seeds often have a temperature range within which they will germinate, and they will not do so above or below this range. Many seeds germinate at temperatures slightly above 60-75 F (16–24 C) [room-temperature in centrally heated houses], while others germinate just above freezing and others germinate only in response to alternations in temperature between warm and cool. Some seeds germinate when the soil is cool 28–40 F (-2 - 4 C), and some when the soil is warm 76-90 F (24–32 C). Some seeds require exposure to cold temperatures (vernalization) to break dormancy. Some seeds in a dormant state will not germinate even if conditions are favorable. Seeds that are dependent on temperature to end dormancy have a type of physiological dormancy. For example, seeds requiring the cold of winter are inhibited from germinating until they take in water in the fall and experience cooler temperatures. Cold stratification is a process that induces the dormancy breaking prior to light emission that promotes germination . Four degrees Celsius is cool enough to end dormancy for most cool dormant seeds, but some groups, especially within the family Ranunculaceae and others, need conditions cooler than -5 C. Some seeds will only germinate after hot temperatures during a forest fire which cracks their seed coats; this is a type of physical dormancy.
Most common annual vegetables have optimal germination temperatures between 75–90 F (24–32 C), though many species (e.g. radishes or spinach) can germinate at significantly lower temperatures, as low as 40 F (4 C), thus allowing them to be grown from seeds in cooler climates. Suboptimal temperatures lead to lower success rates and longer germination periods.
* Light or darkness can be an environmental trigger for germination and is a type of physiological dormancy. Most seeds are not affected by light or darkness, but many photoblastic seeds, including species found in forest settings, will not germinate until an opening in the canopy allows sufficient light for the growth of the seedling.
* Scarification mimics natural processes that weaken the seed coat before germination. In nature, some seeds require particular conditions to germinate, such as the heat of a fire (e.g., many Australian native plants), or soaking in a body of water for a long period of time. Others need to be passed through an animal's digestive tract to weaken the seed coat enough to allow the seedling to emerge.
Dormancy
Some live seeds are dormant and need more time, and/or need to be subjected to specific environmental conditions before they will germinate. Seed dormancy can originate in different parts of the seed, for example, within the embryo; in other cases the seed coat is involved. Dormancy breaking often involves changes in membranes, initiated by dormancy-breaking signals. This generally occurs only within hydrated seeds. Factors affecting seed dormancy include the presence of certain plant hormones, notably abscisic acid, which inhibits germination, and gibberellin, which ends seed dormancy. In brewing, barley seeds are treated with gibberellin to ensure uniform seed germination for the production of barley malt.
Seedling establishment
In some definitions, the appearance of the radicle marks the end of germination and the beginning of "establishment", a period that utilizes the food reserves stored in the seed. Germination and establishment as an independent organism are critical phases in the life of a plant when they are the most vulnerable to injury, disease, and water stress. The germination index can be used as an indicator of phytotoxicity in soils. The mortality between dispersal of seeds and completion of the establishment can be so high that many species have adapted to produce large numbers of seeds.
Details
Germination is the sprouting of a seed, spore, or other reproductive body, usually after a period of dormancy. The absorption of water, the passage of time, chilling, warming, oxygen availability, and light exposure may all operate in initiating the process.
In the process of seed germination, water is absorbed by the embryo, which results in the rehydration and expansion of the cells. Shortly after the beginning of water uptake, or imbibition, the rate of respiration increases, and various metabolic processes, suspended or much reduced during dormancy, resume. These events are associated with structural changes in the organelles (membranous bodies concerned with metabolism), in the cells of the embryo.
Germination sometimes occurs early in the development process; the mangrove (Rhizophora) embryo develops within the ovule, pushing out a swollen rudimentary root through the still-attached flower. In peas and corn (maize) the cotyledons (seed leaves) remain underground (e.g., hypogeal germination), while in other species (beans, sunflowers, etc.) the hypocotyl (embryonic stem) grows several inches above the ground, carrying the cotyledons into the light, in which they become green and often leaflike (e.g., epigeal germination).
Seed dormancy
Dormancy is brief for some seeds—for example, those of certain short-lived annual plants. After dispersal and under appropriate environmental conditions, such as suitable temperature and access to water and oxygen, the seed germinates, and the embryo resumes growth.
The seeds of many species do not germinate immediately after exposure to conditions generally favourable for plant growth but require a “breaking” of dormancy, which may be associated with change in the seed coats or with the state of the embryo itself. Commonly, the embryo has no innate dormancy and will develop after the seed coat is removed or sufficiently damaged to allow water to enter. Germination in such cases depends upon rotting or abrasion of the seed coat in the gut of an animal or in the soil. Inhibitors of germination must be either leached away by water or the tissues containing them destroyed before germination can occur. Mechanical restriction of the growth of the embryo is common only in species that have thick, tough seed coats. Germination then depends upon weakening of the coat by abrasion or decomposition.
In many seeds the embryo cannot germinate even under suitable conditions until a certain period of time has lapsed. The time may be required for continued embryonic development in the seed or for some necessary finishing process—known as afterripening—the nature of which remains obscure.
The seeds of many plants that endure cold winters will not germinate unless they experience a period of low temperature, usually somewhat above freezing. Otherwise, germination fails or is much delayed, with the early growth of the seedling often abnormal. (This response of seeds to chilling has a parallel in the temperature control of dormancy in buds.) In some species, germination is promoted by exposure to light of appropriate wavelengths. In others, light inhibits germination. For the seeds of certain plants, germination is promoted by red light and inhibited by light of longer wavelength, in the “far red” range of the spectrum. The precise significance of this response is as yet unknown, but it may be a means of adjusting germination time to the season of the year or of detecting the depth of the seed in the soil. Light sensitivity and temperature requirements often interact, the light requirement being entirely lost at certain temperatures.
Seedling emergence
Active growth in the embryo, other than swelling resulting from imbibition, usually begins with the emergence of the primary root, known as the radicle, from the seed, although in some species (e.g., the coconut) the shoot, or plumule, emerges first. Early growth is dependent mainly upon cell expansion, but within a short time cell division begins in the radicle and young shoot, and thereafter growth and further organ formation (organogenesis) are based upon the usual combination of increase in cell number and enlargement of individual cells.
Until it becomes nutritionally self-supporting, the seedling depends upon reserves provided by the parent sporophyte. In angiosperms these reserves are found in the endosperm, in residual tissues of the ovule, or in the body of the embryo, usually in the cotyledons. In gymnosperms food materials are contained mainly in the female gametophyte. Since reserve materials are partly in insoluble form—as starch grains, protein granules, lipid droplets, and the like—much of the early metabolism of the seedling is concerned with mobilizing these materials and delivering, or translocating, the products to active areas. Reserves outside the embryo are digested by enzymes secreted by the embryo and, in some instances, also by special cells of the endosperm.
In some seeds (e.g., castor beans) absorption of nutrients from reserves is through the cotyledons, which later expand in the light to become the first organs active in photosynthesis. When the reserves are stored in the cotyledons themselves, these organs may shrink after germination and die or develop chlorophyll and become photosynthetic.
Environmental factors play an important part not only in determining the orientation of the seedling during its establishment as a rooted plant but also in controlling some aspects of its development. The response of the seedling to gravity is important. The radicle, which normally grows downward into the soil, is said to be positively geotropic. The young shoot, or plumule, is said to be negatively geotropic because it moves away from the soil; it rises by the extension of either the hypocotyl, the region between the radicle and the cotyledons, or the epicotyl, the segment above the level of the cotyledons. If the hypocotyl is extended, the cotyledons are carried out of the soil. If the epicotyl elongates, the cotyledons remain in the soil.
Light affects both the orientation of the seedling and its form. When a seed germinates below the soil surface, the plumule may emerge bent over, thus protecting its delicate tip, only to straighten out when exposed to light (the curvature is retained if the shoot emerges into darkness). Correspondingly, the young leaves of the plumule in such plants as the bean do not expand and become green except after exposure to light. These adaptative responses are known to be governed by reactions in which the light-sensitive pigment phytochrome plays a part. In most seedlings, the shoot shows a strong attraction to light, or a positive phototropism, which is most evident when the source of light is from one direction. Combined with the response to gravity, this positive phototropism maximizes the likelihood that the aerial parts of the plant will reach the environment most favourable for photosynthesis.
Additional Information
Seed germination is the initial step in the life cycle of plants, which begins when the inactive dry seed imbibes water and is completed with the protrusion of the radicle from the seed coat. Seed germination is a complex process, which involves several signals and is influenced by both intrinsic and extrinsic factors. Intrinsic factors include seed dormancy and available food stores while water, temperature, oxygen, light, relative humidity, chemicals in the seed environment, and substrate used constitute extrinsic factors. The germination process plays a key role in the domestication of crops as lack of uniform seed germination can result in poor stand establishment, which affects overall crop yield. Germination is largely affected by the balance of phytohormones, especially the abscisic acid (ABA) and gibberellins (GA) ratio. The processes involved in seed germination can be categorized into three prominent stages:
• Phase I, rapid imbibition of water by the dry seed;
• Phase II, metabolism reactivation, including mobilization of food reserves and protein synthesis; and
• Phase III, radicle protrusion.
#16 Dark Discussions at Cafe Infinity » Children Quotes - XVII » 2024-11-23 16:41:37
- Jai Ganesh
- Replies: 0
Children Quotes - XVII
1. Studies have identified a significant 'skills gap' between what students are currently being taught and the skills employers are seeking in today's global economy. Our children must be better prepared than they are now to meet the future challenges of our ever-changing world. - Stephen Covey
2. Crowded classrooms and half-day sessions are a tragic waste of our greatest national resource - the minds of our children. - Walt Disney
3. Most farmers know that their children's future will probably not be in agriculture, but they have a hard time imagining a different life. - Abhijit Banerjee
4. Men fear death as children fear to go in the dark; and as that natural fear in children is increased by tales, so is the other. - Francis Bacon
5. This is the moment when we must come together to save this planet. Let us resolve that we will not leave our children a world where the oceans rise and famine spreads and terrible storms devastate our lands. - Barack Obama
6. I have known plenty of people who, in their later years, had the energy of children and the kind of curiosity and fascination with things like little children. I think we can keep that, and I think it's important to keep that part of staying young. But I also think it's great fun growing old. - Johnny Depp
7. El Salvador, Guatemala, Honduras have all agreed to send additional consular officers from Guatemala, from Honduras, from El Salvador, send them to the U.S. border so that we can more quickly and humanely identify unaccompanied children and process their individual removal. - Joe Biden
8. Even if I was to give my children a small part of my wealth, it would be more than they can digest in many lifetimes. - Azim Premji.
#17 Re: Jai Ganesh's Puzzles » General Quiz » 2024-11-23 16:14:27
Hi,
#10115. What does the term in Biology Ribosome mean?
#10116. What does the term in Biology Ribonucleic acid (RNA) mean?
#18 Re: Jai Ganesh's Puzzles » Doc, Doc! » 2024-11-23 15:37:23
Hi,
#2740. What does the medical term Inferior epigastric artery mean?
#19 Re: Jai Ganesh's Puzzles » 10 second questions » 2024-11-23 15:23:19
Hi,
#9391.
#20 Jokes » Algebra Jokes - VIII » 2024-11-23 15:08:39
- Jai Ganesh
- Replies: 0
Teacher: Why did your mother and father do your algebra homework?
Student: They really understand parent functions.
* * *
Teacher: What is 2n plus 2n?
Student: I don't know. It sounds 4n (foreign) to me.
* * *
Teacher: Let's find the square root of 1 million.
Student: Don't you think that's a bit too radical?
* * *
Surgeon: Nurse! I have so many patients! Who do I work on first?
Nurse: Simple. Use the order of operations.
* * *
Teacher: Your behavior reminds me of square root of 2.
Student: Why?
Teacher: Because its' completely irrational.
* * *
#21 Re: Jai Ganesh's Puzzles » Oral puzzles » 2024-11-23 14:25:07
Hi,
#5907.
#22 Re: Exercises » Compute the solution: » 2024-11-23 13:58:01
Hi,
2238.
#23 Re: Dark Discussions at Cafe Infinity » crème de la crème » 2024-11-22 23:35:46
2099) Albert Fert
Gist:
Work
When materials are reduced to just a few atomic layers—a few nanometers in thickness—their properties change. Independently of one another, Albert Fert and Peter Grünberg discovered the phenomenon Giant Magneto Resistance (GMR) in 1988. GMR involves small changes in magnetic fields creating major differences in electrical resistance. It has also had an impact on electronics, especially read heads, where information stored magnetically is converted to electric current. Thanks to GMR, hard drives have become much smaller.
Summary
Albert Fert (born March 7, 1938, Carcassonne, France) is a French scientist who, with Peter Grünberg, received the 2007 Nobel Prize for Physics for his independent codiscovery of giant magnetoresistance.
Fert received master’s degrees in mathematics and physics from the École Normale Supérieure in Paris in 1962. He earned a doctorate in physical science from the Université Paris-Sud in 1970 and was made a professor there in 1976. He served as research director for the university’s condensed-matter physics laboratory (1970–95) before moving to Unité Mixte de Physique—a laboratory jointly operated by the Université Paris-Sud and the technology firm Thales.
The principle of magnetoresistance was discovered by Lord Kelvin in 1857 when he observed that a conductor’s electrical resistance could be altered by exposing it to an external magnetic field. Fert found that he could drastically increase the resistance within a system by alternating nanometre-thick layers of magnetic and nonmagnetic materials. While this technique was initially too expensive for commercial application, it became an industry-standard manufacturing process for magnetic storage devices such as computer hard drives and portable media players.
Details
Albert Fert (born 7 March 1938) is a French physicist and one of the discoverers of giant magnetoresistance which brought about a breakthrough in gigabyte hard disks. Currently, he is an emeritus professor at Paris-Saclay University in Orsay, scientific director of a joint laboratory (Unité mixte de recherche) between the Centre national de la recherche scientifique (National Scientific Research Centre) and Thales Group, and adjunct professor at Michigan State University. He was awarded the 2007 Nobel Prize in Physics together with Peter Grünberg.
Biography
In 1962 Albert Fert graduated from the École Normale Supérieure in Paris, where he attended courses by the physicists Alfred Kastler and Jacques Friedel. (As an undergraduate he had strong interests in photography and cinema, and was a great admirer of the work of Ingmar Bergman.)
After the École Normale Supérieure, Fert attended the University of Grenoble and in 1963 received his Ph.D. (doctorat de troisième cycle) from the University of Paris with a thesis prepared in the fundamental electronic Orsay Faculty of Sciences and in the physical spectrometry laboratory of the University of Grenoble Faculty of Sciences.
On his return from military service in 1965, Fert became assistant professor at the Orsay Faculty of Sciences of the University of Paris XI (Université Paris-Sud). Under the direction of Ian Campbell at the Laboratory of Solid Physics he prepared for a doctorate Sc.D. (doctorat des sciences) in Physical Sciences on the electrical transport properties of nickel and iron, which he completed in 1970. He was named professor in 1976.
Fert worked as research director for the university's condensed-matter physics laboratory (1970–1995) prior to moving to Unité Mixte de Physique, a laboratory jointly run by the Université Paris-Sud and the technology company Thales.
In 1988, Albert Fert at Orsay in France, and Peter Gruenberg at Jülich in Germany, simultaneously and independently discovered giant magnetoresistance (GMR) in magnetic multilayers. This discovery is considered to mark the birth of spintronics, a new subfield of electronics that exploits not only the electric charge of the electrons but also their magnetism (associated with their intrinsic angular momentum, or spin). Spintronics has already contributed important applications; the introduction of GMR read heads in hard disks has led to a considerable increase in the density of information storage. Other spintronic properties are exploited in magnetic random access memory (MRAM), which may soon impact computer and phone technology. In 2007, Fert and Prof. Grünberg jointly received the Japan Award (300.000 Euro) for their discovery of GMR.
In October 2006, Professor Fert received an honorary doctorate from the Department of Physics of the University of Kaiserslautern.
Fert has made many contributions to the development of spintronics. Following his 2007 Nobel Prize, he began to explore possible spintronics applications of topological properties at surfaces and interfaces. His most recent works are on the topologically protected magnetic solitons called skyrmions and on the conversion between charge current and spin current by topological insulators.

#24 Re: Jai Ganesh's Puzzles » General Quiz » 2024-11-22 23:12:10
Hi,
#10113. What does the term in Geography Rivulet mean?
#10114. What does the term in Geography Riparian water rights mean?
#25 Re: Jai Ganesh's Puzzles » Doc, Doc! » 2024-11-22 22:51:18
Hi,
#2739. What does the medical term Ecthyma mean?