Math Is Fun Forum
You are not logged in.
- Topics: Active | Unanswered
Pages: 1
#1 2024-05-27 22:47:14
- Jai Ganesh
- Administrator

- Registered: 2005-06-28
- Posts: 48,424
Decantation
Decantation
Gist
Decantation is the process of separation of liquid from solid and other immiscible (non-mixing) liquids, by removing the liquid layer at the top from the layer of solid or liquid below. The process can be carried out by tilting the mixture after pouring out the top layer.
Details
Decantation is a process for the separation of mixtures of immiscible liquids or of a liquid and a solid mixture such as a suspension. The layer closer to the top of the container—the less dense of the two liquids, or the liquid from which the precipitate or sediment has settled out—is poured off, leaving denser liquid or the solid behind. The process typically is unable to remove all of the top layer, meaning the separation is incomplete or at least one of the two separated components is still contaminated by the other one.
Processes
Immiscible liquid separation
Decantation can be used to separate immiscible liquids that have different densities. For example, when a mixture of water and oil is present in a beaker, after some time a distinct layer between the two liquids is formed, with the oil layer floating on top of the water layer. This separation can be done by pouring oil out of the container, leaving water behind. Generally, this technique gives an incomplete separation as it is difficult to pour off all of the top layer without pouring out some parts of the bottom layer.
A separatory funnel is an alternative apparatus for separating liquid layers. It has a valve at the bottom to allow draining off the bottom layer. It can give better separation between the two liquids.
Liquid-solid separation
Decantation can also separate solid and liquid mixtures by allowing gravity to pull the solid fragments to settle at the bottom of the container. In laboratory situations, decantation of mixtures containing solids and liquids occur in test tubes. To enhance productivity, test tubes should be placed at a 45° angle to allow sediments to settle at the bottom of the apparatus.
A centrifuge machine may also be used in decantation as the natural process of settling down is time-consuming and tedious. A centrifuge forces the precipitate to the bottom of the container; if the force is high enough, solids can aggregate to form pellets, making it easier to separate the mixtures. Then the liquid can be more easily poured away, as the precipitate will tend to remain in its compressed form.
A decanter centrifuge may be used for continuous solid-liquid separation.
Examples
Decantation is frequently used to purify a liquid by separating it from a suspension of insoluble particles (e.g. in red wine, where the wine is decanted from the potassium bitartrate crystals to avoid unsavory taste). This makes the wine more tonic and astringent.
Cream accelerates to the top of milk, allowing the separation of milk and cream. This is used in the cheese industry. Fat is determined in butter by decantation.
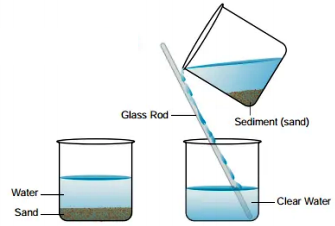
To obtain a sample of clear water from muddy water, muddy water is poured into another container, which separates the water from the mud.
In the sugar industry, the processing of sugar beets into granular sugar involves many liquid–solid separations; e.g. separation of syrups from crystals.
Decantation is also present in nanotechnology. In the synthesis of high quality silver nanowire (AgNW) solutions and fabrication process of high performance electrodes, decantation is also being applied which greatly simplifies the purification process.
After using a desiccant to absorb water from an organic liquid, the organic liquid can often be decanted away from the desiccant.
The process of deriving vinegar also requires decantation to remove fats and biomolecular antioxidants from the raw substance.
Plasma can be separated from blood through decantation by using a centrifuge.
Mercury is disposed of in water bodies during mining, turning the water unfit and toxic. The mercury can be removed through decantation.
Additional Information
Decantation is a process of separation of liquid from solid and other immiscible liquids by removing the liquid layer at the top from the layer of liquid or solid below. It is a process deeply rooted in the tradition of winemaking. The decantation process is also used in our household by our mothers to remove smaller impurities of mud and dust from rice and pulse using water.
In general, the process of separation of solid impurities from the liquid solution is termed decantation.
Decantation is a process used for the separation of mixtures of immiscible liquids or of a liquid and a solid mixture such as a suspension. Generally, in decantation liquid is separated from sediments or another immiscible liquid with different densities manually.
Decantation is a method of Separation of Mixtures. This method is used in separating immiscible such as oil and water. The mixture of oil and water forms two different layers where water is at the bottom and oil is at the top this mixture is separated using the decantation process.
Decantation Definition
Decantation is a process of separating two immiscible liquids or a liquid and solid in which solid is suspended in the liquid. This process uses tilting and draining lighter liquid in another vessel. The mud settled at the bottom of the water is separated using the decantation process.
Decantation Process of Separating Mixtures
Mixtures can be easily separated using the decantation process.
Decantation Process
Decantation process of separating mixtures involves pouring the mixture into a container and allowing it to settle. The layer closer to the top of the container, which is less dense than the two, is then carefully poured off, leaving the denser layer behind. In the case of solid and liquid mixtures, Decantation is done by allowing gravity to pull the solid fragments to settle at the bottom of the container.
Types of Decantation
It can be categorized into two main types based on the components being separated:
Immiscible Liquid Separation
This type of decantation involves separating two immiscible liquids, which are unable to mix. An example of this is the separation of water and oil. When a mixture of water and oil is present in a container, a distinct layer between the two liquids forms over time, with the oil layer floating on top of the water layer. Decantation can be used to separate these immiscible liquids by pouring the oil out of the container, leaving the water behind
Solid-Liquid Separation
Decantation can be used to separate solid and liquid mixtures by allowing gravity to pull the solid fragments to settle at the bottom of the container. In laboratory situations, decantation of mixtures containing solids and liquids occurs in test tubes, with the test tubes often being placed at a 45° angle to allow sediments to settle at the bottom of the apparatus.
It appears to me that if one wants to make progress in mathematics, one should study the masters and not the pupils. - Niels Henrik Abel.
Nothing is better than reading and gaining more and more knowledge - Stephen William Hawking.
Offline
Pages: 1